A Vancouver Island woman living with advanced Parkinson’s disease cannot understand why a potentially life-saving therapy is out of reach for patients in British Columbia.
Paddi Wood has tried a number of treatments and drugs since she was diagnosed with the degenerative neurological condition in 2008. She says she swallows 72 pills per day to ease her shaking, tremors, and involuntary movements -- an exhausting yet ineffective regimen that regularly leaves her writhing in pain without relief.
The 68-year-old believes a treatment called Duodopa is her only hope. However, the province refuses to cover the cost of the $60,000 per year remedy, saying there is no clinical evidence to support claims it reduces mortality.
“We have tried everything imaginable,” Wood told CTV Vancouver Island on Friday.
Parkinson's affects one in every 500 people in Canada. Over 100,000 Canadians are living with the disease, and approximately 6,600 new cases are diagnosed in Canada each year, according to researchers at the University of British Columbia.
The tremors, muscle stiffness, lack of balance, and involuntary movements associated with Parkinson’s are caused by a lack of dopamine in the brain.
According to Duodopa’s manufacturer AbbVie Inc., the treatment reduces those disabling motor symptoms and improves the patient’s ability to perform daily activates by delivering a steady stream of dopamine boosting gel through a pump-powered tube into the small intestine.
The pump is believed to provide far more consistent relief than Wood’s oral medications.
“Nothing works,” said Wood. “Why can’t we try the Duodopa pump? It might keep me alive.”
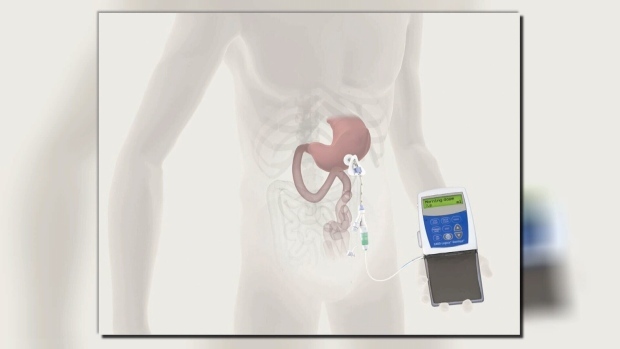
The treatment is approved by Health Canada and covered in several provinces including Ontario and Alberta.
“It is life-changing and in some cases life-saving for these people,” said Parkinson Society British Columbia CEO Jean Blake.
She says AbbVie has proved the treatment works in advanced patients, but is unwilling to submit additional research in order see it approved in B.C.
“BC PharmaCare has a policy that the manufacturer must submit any new evidence to a national process called Common Drug Review.”
According to Blake, “the manufacturer is not willing to do that because they have secured agreements with five other jurisdictions.”
That’s of little reassurance to Wood and her family.
“In Canada. we have a history of looking after the most vulnerable, and she is certainly one of them,” said Wood’s husband Brian.
He’s considered selling their house to pay for the treatment, but estimates that would only cover the next five years.
“How much longer can she go on like this?” he said. “I’m not optimistic.”
Wood says the disease has taken an immense toll on her well-being. Her condition has left her so frail that she has been mistaken for Brian’s mother.
“I’ve lost so much weight that I’m considered grossly malnourished,” she said. “We’ve tried all these damned other things that the government insist we try before they would approve the pump. We’ve tried them all, nothing works.”
Wood plans to continue her fight in the hopes someone will listen.
“I don’t want to die. Not yet. I’m not old enough.
With a report from CTV Vancouver Island’s Chandler Grieve